Abstract
Background: PD-1 inhibitors play a key role in the treatment of relapsed or refractory (R/R) classical Hodgkin lymphoma (cHL) but improving upon current treatment responses remain of clinical interest. Lymphocyte-activation gene 3 (LAG-3) is involved in T-cell regulation and is commonly coexpressed with PD-1 on anergic T cells. Dual blockade of PD-1 and LAG-3 has demonstrated antitumor activity leading to US Food and Drug Administration approval for the treatment of patients with unresectable or metastatic melanoma. Favezelimab (MK-4280), a humanized IgG4 LAG-3 inhibitor, plus pembrolizumab (anti-PD-1) is being investigated in the multicohort phase 1/2 MK-4280-003 efficacy and safety study (NCT03598608) in patients with R/R hematologic malignancies. Initial results from the MK-4280-003 study demonstrated promising antitumor activity and acceptable safety in anti-PD-1-naive patients with R/R cHL (cohort 1) (Johnson NA, et al. J Clin Oncol. 2022;40(16_suppl):7516). Updated results from cohort 1 after additional follow-up are presented.
Methods: Part 1 was the safety lead-in phase to determine the recommended phase 2 dose (RP2D) followed by a dose-expansion phase (part 2). Eligible patients in cohort 1 had R/R cHL after autologous stem cell transplantation (ASCT) or were ineligible for ASCT, and had no prior anti-PD-1 therapy. Part 1 included patients from all cohorts who received escalating doses (per modified toxicity probability index interval design) of pembrolizumab IV 200 mg Q3W and favezelimab IV 200 mg or 800 mg Q3W. In part 2, patients received pembrolizumab plus favezelimab at the established RP2D (800 mg Q3W) for ≤35 cycles (~2 years). CT scans were performed every Q12W and PET scans at Weeks 12 and 24 to assess response by investigator review per the IWG 2007 criteria. Adverse events (AE) were graded per the NCI CTCAE, v4.0. The primary end points were safety and RP2D. Objective response rate (ORR) was a secondary end point. Duration of response (DOR), progression-free survival (PFS), and overall survival (OS) were exploratory end points.
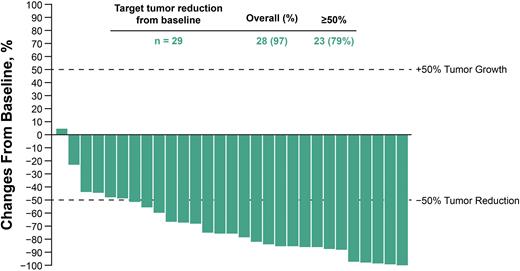
Results: Among the 30 patients enrolled in cohort 1, the median age was 40.5 years, 53% had ECOG PS 0, and 80% had ≤3 prior lines of therapy. At database cutoff (May 03, 2022), 6 (20%) patients had completed 2 years of study treatment and 13 (43%) patients had discontinued (9 progressive disease; 4 AEs); 4 (13%) patients discontinued due to treatment-related adverse events [TRAEs]); 11 (37%) patients had treatment ongoing at the time of database cutoff. No deaths were treatment-related. Among the 26 patients (87%) who had TRAEs; the most common (≥10%) were hypothyroidism (27%); infusion-related reaction (23%), fatigue (20%); pruritus and headache (17% each), chills (13%); arthralgia, hyperthyroidism, maculopapular rash, myalgia, nausea and pyrexia (10%, each). Grade 3 or 4 TRAEs occurred in 7 patients (23%); including 1 (3%) event each of thrombocytopenia, enterocolitis, fatigue, autoimmune hepatitis, increased blood creatine phosphokinase, increased amylase, increased lipase, organizing pneumonia, and maculopapular rash. Of 3 patients who received hematopoietic stem cell transplantation after completion of study treatment, 1 had a grade 3 or 4 AE unrelated to study treatment (increased blood bilirubin) that resolved. After a median follow-up of 15.5 months, 22 patients had objective response (ORR, 73% [95% CI, 54-88]; complete response, 9 [30%]; partial response, 13 [43%]). Of 29 patients (97%) who received a postdose scan, 28 had a baseline reduction in target lesions and 23 (79%) had ≥50% reduction from baseline (Figure). Median DOR was not reached (NR; range, 2.6-28.8+ months); 7 patients (56%) had response ≥12 months. Median PFS was 19.4 months (95% CI, 8.5-NR); 12-month PFS rate was 62%. Median OS was NR (95% CI, NR-NR); 12-month OS rate was 96%.
Conclusion: After additional follow-up, favezelimab plus pembrolizumab combination therapy continued to demonstrate sustained antitumor activity and acceptable safety in anti-PD-1-naive patients with R/R cHL. Further studies comparing the activity of this combination to that of single-agent pembrolizumab would be of clinical benefit.
Disclosures
Johnson:Merck, AbbVie, Roche, Gilead: Consultancy. Borchmann:Novarts: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Miltenyi Biotec: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Gregory:Roche, Novartis, BMS, Janssen: Consultancy; Roche, BMS: Honoraria; Janssen: Other: Expert testimony; Roche, Novartis: Other: Travel/ Accommodations/ Expenses; Roche: Speakers Bureau; BeiGene, Merck, AbbVie, Janssen: Research Funding. Herrera:Merck: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Takeda: Consultancy; Tubulis: Consultancy; Regeneron: Consultancy; Genmab: Consultancy; Pfizer: Consultancy; Caribou: Consultancy; Adicet Bio: Consultancy; KiTE Pharma: Research Funding; Gilead: Research Funding; Karyopharm: Consultancy. Vucinic:Novartis, Gilead Kite, Takeda, MSD, BMS Celgene, Abbvie, Amgen: Honoraria; MSD, BMS Celgene, Novartis, Gilead Kite, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sobi, BMS Celgene: Other: travel, accommodations, expenses. Armand:Tensha: Research Funding; Pfizer: Consultancy; Regeneron: Consultancy; BMS: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding; Roche: Research Funding; Genmab: Consultancy; Xencor: Consultancy; C4: Consultancy; Enterome: Consultancy; Tessa: Consultancy; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy; Epizyme: Consultancy; Miltenyi: Consultancy; Daiichi Sankyo: Consultancy; Morphosys: Consultancy; Celgene: Consultancy; ADC Therapeutics: Consultancy; Infinity: Consultancy; Adaptive: Consultancy, Research Funding; Affimed: Consultancy, Research Funding; Otsuka: Research Funding; Sigma Tau: Research Funding; IGM: Research Funding; Kite: Research Funding. Avigdor:Takeda, Gilead, Novartis, Roche, BMS: Consultancy; AbbVie: Honoraria. Gasiorowski:Otsuka: Honoraria; MSD: Honoraria; Novartis: Honoraria; Astellas: Honoraria; AbbVie: Honoraria; Janssen: Honoraria; Antengene: Honoraria. Herishanu:Janssen, AbbVie, Roche, Medison, BeiGene: Honoraria. Keane:Roche, Beigene, MSD: Consultancy. Kuruvilla:Antengene: Consultancy; Janssen: Honoraria; Astra Zeneca: Honoraria, Research Funding; Amgen: Honoraria; Abbvie: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Novartis: Honoraria; Karyopharm: Consultancy, Honoraria, Other: DSMB; Merck: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Inctye: Honoraria; Medison Ventures: Consultancy; Seattle Genetics: Consultancy, Honoraria; Pfizer: Honoraria; Lymphoma Canada: Membership on an entity's Board of Directors or advisory committees. Palcza:Merck & Co., Inc.: Current Employment. Pillai:Merck & Co., Inc.: Current Employment, Current holder of stock options in a privately-held company. Nahar:Merck & Co., Inc.: Current Employment. Timmerman:Bristol Myers Squibb: Research Funding; Merck: Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Oncovalent: Consultancy; A2: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal